WHAT IS GRAPHITE?
A naturally-occurring stable allotrope of carbon, graphite is the product of the transfiguration of carbonaceous sediments or the crystallization of magmatic carbon and commonly occurs as large masses or in vein formations on crystalline rocks such as gneiss, marble, and schist under high temperatures and pressure
CHEMICAL AND PHYSICAL PROPERTIES
Graphite is chemically composed of layers of interconnected hexagonal rings of carbon atoms, which are known as graphene, which gives it a tendency to crystallize in a hexagonal manner.
It is a chemically inert allotrope of carbon with a greyish-black appearance that offers a multitude of advantageous physical and chemical properties, which include :
An excellent electric and thermal conductor - given that each carbon atom forms three covalent bonds with each other for its hexagonal arrangement, it implies that each carbon atom contributes to a delocalized electron, giving it its conducting abilities.
An outstanding means of lubrication: the layers of graphene are held together by weak Van der Waals forces, therefore the two-dimensional lattice layers of graphite can be cleaved against with minimal effort. This property also gives it a soft, soapy feel to the touch.
A robust allotrope by nature: graphite is chemically inert and is therefore immune to oxidation and temperature fluctuations; it is also high in density and possesses a melting point of 3650℃, making it a primary material in fireproofing agents.
It is a chemically inert allotrope of carbon with a greyish-black appearance that offers a multitude of advantageous physical and chemical properties, which include :
An excellent electric and thermal conductor - given that each carbon atom forms three covalent bonds with each other for its hexagonal arrangement, it implies that each carbon atom contributes to a delocalized electron, giving it its conducting abilities.
An outstanding means of lubrication: the layers of graphene are held together by weak Van der Waals forces, therefore the two-dimensional lattice layers of graphite can be cleaved against with minimal effort. This property also gives it a soft, soapy feel to the touch.
A robust allotrope by nature: graphite is chemically inert and is therefore immune to oxidation and temperature fluctuations; it is also high in density and possesses a melting point of 3650℃, making it a primary material in fireproofing agents.
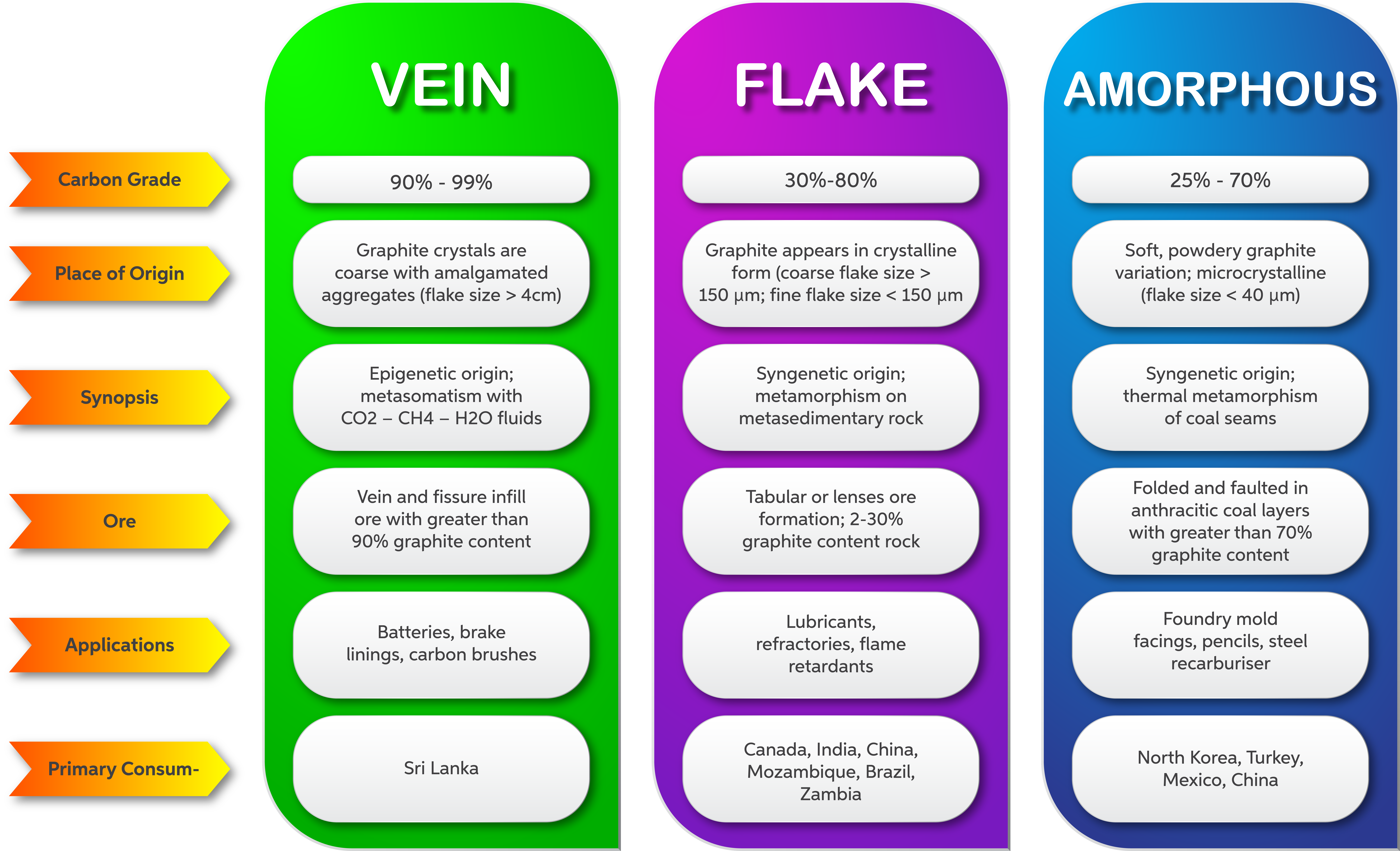
TYPES OF GRAPHITE
Graphite occurs in natural forms across the world in three main variations, namely crystalline vein, flake, and amorphous graphite, and as a man-made form called synthetic graphite
CRYSTALLINE VEIN GRAPHITE
Also known as Ceylon graphite or plumbago, vein graphite is the creation of high-temperature graphitic fluid carbon known as pegmatitic fluids depositing in surrounding ore in the form of fissures or veins. Its main commercial mines are present in Sri Lanka, whilst also being present in the United States and Great Britain.
Vein graphite is infamous for its purity, with Ceylon graphite boasting between 94% - 99% carbon content. It exists as a coarse graphite crystal aggregate with particles greater than 4cm in length and is the most conductive and chemically inert variation when compared with its counterparts.
Crystalline vein graphite is a prominent component in electric vehicle batteries, mobile and electronic applications, energy storage devices and as an extraction source of graphene.
FLAKE GRAPHITE
Flake graphite is the product of carbon subjected to high temperatures and pressure sedimentation on the metasedimentary rock in a uniform manner. This results in a crystalline, flaky form of graphite with flakes being greater than or less than 150µm depending on granularity.
Flake graphite has a purity grade of 30%-80% carbon content and is typically abundant across the world, being sourced from countries such as China, Brazil, India, Canada, Mozambique, and Zambia. It is used in applications such as fuel cells, vanadium redox batteries, and nuclear reactors
AMORPHOUS GRAPHITE
Amorphous graphite is the most abundant form of graphite available naturally and is formed when an anthracite coal seam is met with a metamorphic change such as magma or tectonic stress. This phenomenon gives amorphous graphite its microcrystalline structure and therefore makes it a seam mineral with the poorest carbon grading of 25% to 40%. It is mainly sourced from China, Mexico, North Korea, and Turkey. Amorphous graphite is incorporated into items such as metallurgical furnaces, crucibles, molds, and pencil lead
CRYSTALLINE VEIN GRAPHITE
Also known as Ceylon graphite or plumbago, vein graphite is the creation of high-temperature graphitic fluid carbon known as pegmatitic fluids depositing in surrounding ore in the form of fissures or veins. Its main commercial mines are present in Sri Lanka, whilst also being present in the United States and Great Britain.
Vein graphite is infamous for its purity, with Ceylon graphite boasting between 94% - 99% carbon content. It exists as a coarse graphite crystal aggregate with particles greater than 4cm in length and is the most conductive and chemically inert variation when compared with its counterparts.
Crystalline vein graphite is a prominent component in electric vehicle batteries, mobile and electronic applications, energy storage devices and as an extraction source of graphene.
FLAKE GRAPHITE
Flake graphite is the product of carbon subjected to high temperatures and pressure sedimentation on the metasedimentary rock in a uniform manner. This results in a crystalline, flaky form of graphite with flakes being greater than or less than 150µm depending on granularity.
Flake graphite has a purity grade of 30%-80% carbon content and is typically abundant across the world, being sourced from countries such as China, Brazil, India, Canada, Mozambique, and Zambia. It is used in applications such as fuel cells, vanadium redox batteries, and nuclear reactors
AMORPHOUS GRAPHITE
Amorphous graphite is the most abundant form of graphite available naturally and is formed when an anthracite coal seam is met with a metamorphic change such as magma or tectonic stress. This phenomenon gives amorphous graphite its microcrystalline structure and therefore makes it a seam mineral with the poorest carbon grading of 25% to 40%. It is mainly sourced from China, Mexico, North Korea, and Turkey. Amorphous graphite is incorporated into items such as metallurgical furnaces, crucibles, molds, and pencil lead
WHAT IS GRAPHENE?
Graphene are the single isolated sheets of hexagonally arranged carbon lattices which make up the structure of graphite. These atom-thick sheets join together via Van der Waals forces to form graphite.
SYNTHETIC GRAPHITE
This variation of graphite is the product of subjecting amorphous carbon materials such as coal tar pitch and calcined petroleum coke to extreme temperatures, with the graphitization process achieved with the aid of electric current.
As the process converts the amorphous carbon structures into uniform graphite lattices, synthetic graphite mirrors the properties of naturally occurring varieties, such as high electric and thermal conductivity and high crystallinity.
Given its properties, synthetic graphite is essential in applications such as lubricants, electrodes, and refractory linings.
As the process converts the amorphous carbon structures into uniform graphite lattices, synthetic graphite mirrors the properties of naturally occurring varieties, such as high electric and thermal conductivity and high crystallinity.
Given its properties, synthetic graphite is essential in applications such as lubricants, electrodes, and refractory linings.
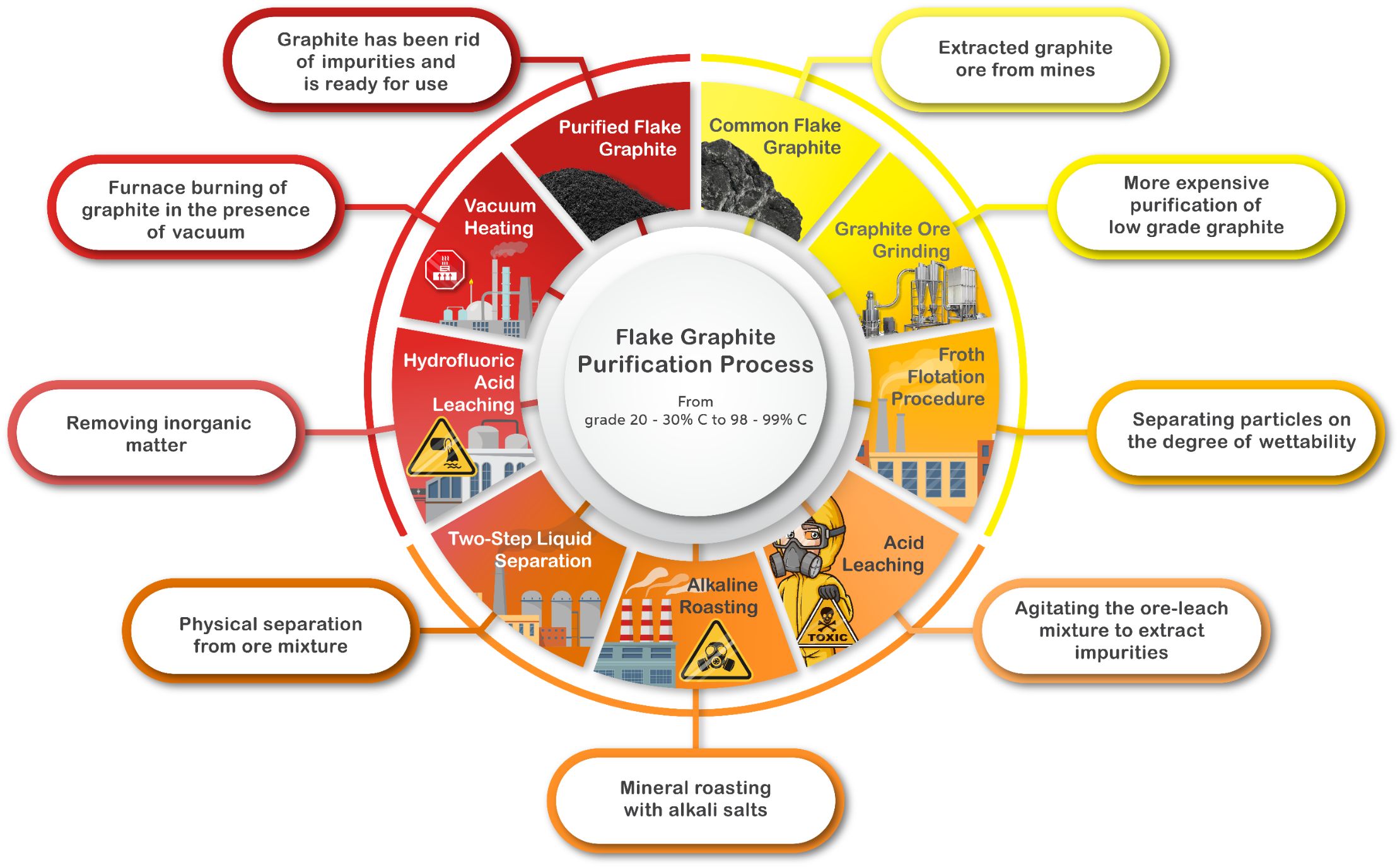
Flake Graphite Purification Process
The purification process of flake graphite is an intense procedure that involves a massive amount of energy for extraction and processing of the ore to reach 99% carbon grade - the flake graphite mine as a whole is an expensive investment and its extraction process, surface mining, causes great short-term and long-term environmental damage.
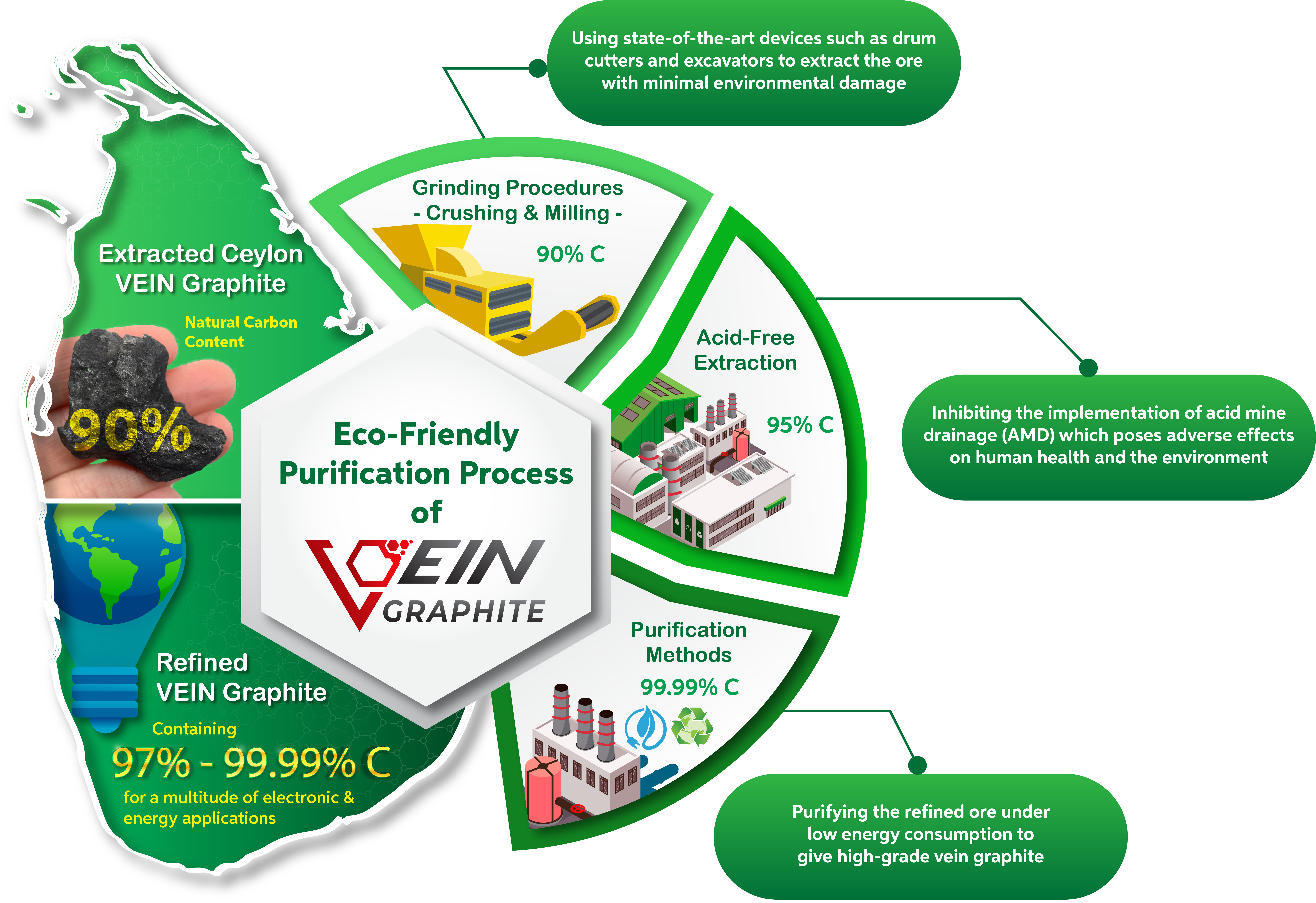
Vein Graphite Purification Process
Vein graphite ore at initial extraction commonly bears a carbon grade of 97% - 99%, therefore it needs far less processing and purification. The vein graphite mining procedure is far more environmentally friendly, cost-effective, and sustainable for long-term use.
(Pictured here is the processing of graphite ore of 90% carbon grading until it reaches a purity content of 99%)
(Pictured here is the processing of graphite ore of 90% carbon grading until it reaches a purity content of 99%)
VERTICAL MINING ALONG THE VEIN
The vertical mining executed at Dodangaslanda involves excavating along the fissures of the graphite ore embedded hundreds of feet below the Earth’s surface. This form of mining causes minimal damage to the environment and is capable of reaching the deepest resources of graphite in a mine.
Advantages of Vertical Mining

